General Terms in Thermodynamics
General Terms in Thermodynamics: Overview
This topic covers concepts, such as Macroscopic Variables of the System, Microscopic Variables of the System, General Terms in Thermodynamics, Thermodynamic System, System, Surroundings and Boundaries and Isolated System.
Important Questions on General Terms in Thermodynamics
Time taken to heat water upto a temperature of (from room temperature) is and time taken to heat mustard oil (of same mass and at room temperature) upto a temperature of is , then (given mustard oil has smaller heat capacity)
For the process to occur under adiabatic conditions, the correct condition is:
Which is not the microscopic variable of the system?
A system is said to be an isolated system if
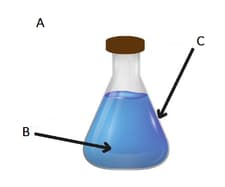
If we want to study the liquid inside the flask, we can define A, B and C as
What is a boundary for a thermodynamic system?
For an isolated system, the entropy:
Which among this is not an exothermic reaction?
What is the equation of entropy?
Entropy occurs due to ______.
An increase in enthalpy leads to an increase in _______.
Which of the following is/are macroscopic variables:
Which of the following is/are macroscopic variables:
Which of the following is/are macroscopic variables:
An open system is one in which
Which of the following parameters can define the thermodynamic state of a system?
An open system is one in which
A gas is filled in a box, which is moving with velocity . The internal energy of the gas will not include kinetic energy:
A system is in a state of thermodynamical equilibrium, if:
Which one of the following is not a macroscopic variable of the gas (system) filled in a container?
